
A drug company is recalling some of its ADHD and narcolepsy medication because the bottles may contain the wrong pills inside.
The U.S. Food and Drug Administration said in a notice published last week that Azurity Pharmaceuticals is recalling one lot of Zenzedi 30-milligram dextroamphetamine sulfate tablets.
The move comes after a pharmacist in Nebraska found carbinoxamine maleate tablets, an antihistamine drug, inside a bottle of Zenzedi tablets.
Zenzedi is a stimulant prescription medicine often used to treat ADHD and narcolepsy by increasing attention and decreasing impulsiveness, according to the National Institute of Health. Carbinoxamine maleate has sedative and drying properties, and is often used to treat seasonal allergies, meaning the two drugs have opposing reactions.
Get top local stories in San Diego delivered to you every morning. Sign up for NBC San Diego's News Headlines newsletter.
“Patients who take carbinoxamine instead of Zenzedi will experience undertreatment of their symptoms, which may result in functional impairment and an increased risk of accidents or injury,” the FDA said.
Adverse effects include drowsiness, sleepiness and other serious conditions including thyroid disorder.
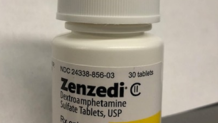
The recalled medication has a lot number F230169A with an expiration date of June 2025. It was shipped to wholesales nationwide between Aug. 23 and Nov. 29, 2023.
Recall Alert
The impacted tablets are light yellow and have a hexagonal shape with “30” embossed on one side and “MIA'' embossed on the other. They come in a white bottle with black lettering. Carbinomaxine Maleate tablets have “GL” imprinted on one side and “211” on the other.
Massachusetts-based Azurity said it has not received any reports of adverse events related to the mishap.
According to the FDA, consumers should contact their doctor or healthcare provider if they've experienced any problems related to taking the recalled medication. Any adverse reactions could also be reported to Azurity by emailing aereports@azurity.com.



