A Chula Vista family who was infected with the new coronavirus is touting the effects of an experimental blood transfusion treatment for saving their daughter's life after her condition started to worsen in the ICU.
Marielena Barajas, her daughter, Maya, her husband and her mother-in-law were sheltering-in-place together when they were struck with an outbreak of COVID-19. While all had symptoms, Maya Barajas' were critical.
Maya Barajas was admitted to Sharp Memorial Hospital's ICU in early April. She was placed on a ventilator and spent 20 days on the machine to help her breathe. She wasn't recovering and her doctor was running out of options, according to Sharp HealthCare's Senior Blood Bank Specialist John Stephan.
Her Doctor suggested treating Maya Barajas with convalescent plasma, which is collected from the blood of people who have already recovered from COVID-19. Their plasma may contain antibodies that can be administered to patients in severe condition to help them recover.
“The patient’s condition, going down very fast, quickly stabilized after the CCP transfusion," Stephan said.
While the therapy is still experimental, the U.S. Food and Drug Administration on March 24 allowed doctors to use plasma under an emergency approval system. Doctors can apply to the FDA to use it for their patients, and the agency will review the requests quickly and make decisions on a case-by-case basis.
The FDA said the practice has not yet been shown to be a safe and effective treatment for COVID-19, but it shows promise. The agency is studying the effect in clinical trials.
Local
About a month after being admitted to the ICU, Maya Barajas remains in the hospital but is was taken off the ventilator. Her mother said she is making progress.
“We can thank God, everyone who prayed, all of the doctors and medical staff as well as the San Diego Blood Bank and donors."
Maya Barajas' transfusion was provided by donations made to the San Diego Blood Bank and Marielena Barajas, seeing the positive effects the plasma had on her daughter, wanted to help others who may be severely affected by COVID-19. She donated her own plasma to support someone else in need.
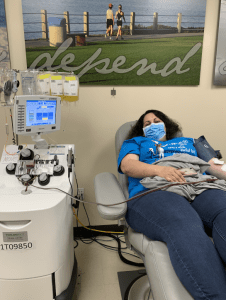
The San Diego Blood Bank began collecting convalescent plasma in early April after the FDA released guidance for jurisdictions that wanted to study the treatment option.
The agency is looking for people who have been confirmed positive by a laboratory test and have been symptom-free for at least 28 days or for people who have been confirmed negative by a second test for at least 14 days. Even those who have not been tested, who are willing to donate plasma, should sign up, the blood bank said.
The method of using antibodies from blood plasma is not new. The concept dates back to the early 20th century and has been successfully used to counter mumps and measles, according to Johns Hopkins University.
The FDA said the method was also tested in other outbreaks including the 2003 SARS outbreak and the 2010 H1N1 outbreak.
According to NBC News, the treatment is not without risks. There is danger in giving a patient the wrong type of blood or inadvertently transmitting other pathogens in a transfusion, but safety advancements over the past two decades have made adverse outcomes rare.



